What is the purpose of this guidance?
This guidance is to be used by IRB staff, members, and investigators as guidance to facilitate the USC IRB review of protocols where a USC Investigator is conducting, supporting, or collaborating with the Department of Defense (DoD).
Where does this guidance come from?
This guidance is based on updates shared with the USC HRPP and reflects the DoD Instruction (DODI) 3216.02, “Protections of Human Subjects and Adherence to Ethical Standards in DOD-Conducted and-Supported Research.”
It might. Studies that seek to enroll DoD-affiliated personnel as participants and/or the data or specimens of DoD-affiliated personnel requires DoD approval and oversight. DoD-affiliated personnel are defined as: Service members, Reserve Service members, National Guard members, DoD Civilians, and DoD Contractors.
USC investigators who intend to conduct research or support or collaborate with the DoD in research activities must go through both the USC IRB review and concurrent DoD Component Office of Human Research Protections (COHRP) review(s) prior to beginning their research.
DoD Component Office of Human Research Protections (COHRP).
DoD COHRPs review studies under a different set of regulations. They are similar and align with IRB regulations. However, some DoD regulations are specific to using DoD personnel. The USC IRB will always ‘honor’ the DoD COHRP review team needs/wants for these specific instances.
Absolutely! Please budget a minimum of two extra months in your process. This will allow for back-and-forth cycles between the DoD COHRP and USC IRB.
YES! In fact, it’s very likely. Each funding source and participant pool may have its own COHRP, and each COHRP may interpret the regulations a little bit differently and have different requests.
The COHRP review should take place concurrently with the USC IRB review process.
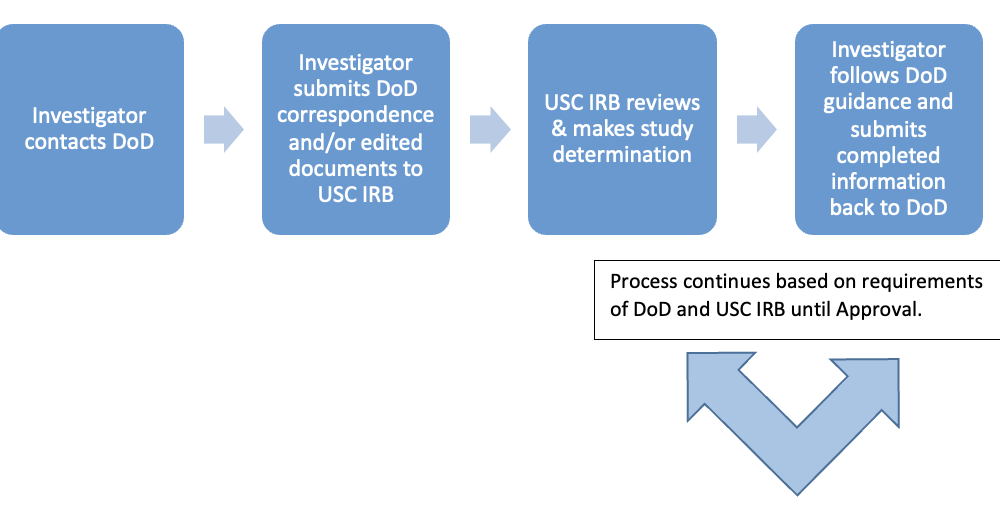
We have outlined the process below!
Initial Submissions
Prior to USC IRB review, identify and reach out to the DoD COHRP(s) associated with each of your funding source(s) and/or participant pool(s) to see what their expectations are for the review process. You may need to create separate USC IRB applications for each component.
Here is a list of some of the COHRPs and their contact information.
- Under Secretary of Defense for Research and Engineering (USD R&E):Human Research Protections (DOHRP) – DCTO (S&T)
- Defense Advanced Research Projects Agency (DARPA) website: Defense Advanced Research Projects Agency (darpa.mil)
- Department of the Airforce:Airforce Component Office for Human Research Protections (includes Air National Guard) (pentagon.af-sg.mbx.afmsa-sge-c@health.mil)
- Department of the Army:Army Human Research Protections Office (includes Army National Guard and Army Reserves) ( ncr.hqda-otsg.mbx.otsg-ahrpo@health.mil)
- Defense Health Agency (DHA):Office of Research Protection (hrpp@health.mil)
- Department of Navy:Human Research Protection Program (Navy, BUMED, Marines): (ncr.bumedfchva.mbx.DON-HRPP@health.mil)
- Defense Threat Reductions Agency (DTRA): Research and Development Directorate(Research and Development (dtra.mil))
- S. Army Medical Research Development Command (USAMRDC):Office of Human Research Oversight (OHRO): (usarmy.detrick.medcom-usamrmc.other.hrpo@health.mil)
- United States Special Operations Command (USSOCOM): Research Protections (hrpp@socom.mil)
- Uniformed Services University of the Health Sciences (USUHS):Human Research Protection Program: (IRB1@usush.edu)
If you have any questions during this process, you can also reach out to the USC IRB for help: hrpp@usc.edu.
Create your USC IRB application(s).
- IMPORTANT: Ensure any COHRP-specific requirements are addressed in the application.
- Examples of these kind of requirements we have seen are required consent language; the requirement for the IRB to document that scientific review has taken place in their approval letter; and the requirement for an ombudsperson to be designated for recruitment and listed in the IRB application (in iStar # 40.1).
- IMPORTANT: Upload any written communications you have had with your COHRP contact to iStar # 40.1.
- IMPORTANT: Include the name(s) and contact information for your COHRP contact(s) in iStar # 40.2 so USC IRB can get in touch with them as needed during the IRB review process.
Submit your USC IRB application(s) for review. Once USC IRB review is complete, the study will be approved with the single remaining contingency of COHRP approval.
Submit the materials reviewed by the USC IRB to the applicable component(s) for the COHRP review.
If the COHRP(s) require changes to the submitted materials, revise your open IRB application(s) accordingly, and submit the revised application(s) back to the USC IRB for review.
- IMPORTANT: Upload the correspondence from the COHRP requesting the changes to iStar # 40.1 so the USC IRB can confirm that the requested changes have been made.
- IMPORTANT: This process will repeat until the COHRP approval has been granted.
Once the COHRP approval is granted, upload your COHRP approval letter to iStar section #40.1, and submit the application(s) back to the USC IRB for review.
Once USC IRB issues their final approval / determination, submit this documentation to all applicable COHRPs for their records.
Is this the end?
Not quite! Let’s talk amendments!
Amendments
USC will want to see any study revisions that impact participant-facing materials or procedures prior to them going into effect.
Additionally, each COHRP will have their own expectations for reporting revisions. These expectations are communicated in the COHRP approval letter.
- IMPORTANT: The letter will explain which kinds of changes should be approved by COHRP first (before being communicated to USC IRB), and which kinds of changes should be approved by USC IRB first (before being communicated to the COHRP).
- IMPORTANT: If you have any questions about the order of communications, check with your COHRP contact before submitting.
- IMPORTANT: DOD agencies have expectations for what changes require a separate IRB application versus what would be revisions to the existing project/scope of work. If you have any questions about whether something (a new procedure, a new site, a new population, a new funding source) should be a new IRB submission or an amendment, check with the COHRP contact before submitting.
Is this the end?
Not quite! Let’s talk reporting responsibilities.
Reporting Responsibilities
USC Investigators conducting, supporting, or collaborating with the DoD (DoD) must report the following to all applicable COHRPs within 30 days of occurrence:
- When significant changes to the research protocol are approved by the IRB:
- Changes to key investigators or institutions.
- Decreased benefit or increased risk to participants in greater than minimal risk research.
- Addition of vulnerable populations as participants.
- Addition of DoD-affiliated personnel as participants.
- Change in status when a previously enrolled participant becomes pregnant, or when the researcher learns that a previously enrolled participant is pregnant, and the protocol was not reviewed and approved by the IRB in accordance with 45 CFR 46, Subpart B.
- Change in status when a previously enrolled participant becomes a prisoner, and the protocol was not reviewed and approved by the IRB/EC in accordance with 32 CFR 219, Subpart C.
- Closure of a DoD-supported study.
Investigators may be subject to additional COHRP-specific reporting requirements, including but not limited to:
- Results of the IRB continuing review.
- Change of reviewing IRB.
- Notification by any federal department, agency or national organization that any part of the HRPP is under investigation for cause involving a DoD-supported research protocol.
- Any suspension or termination of DoD-supported research.
IMPORTANT: If you have any questions about whether something should be reported to applicable COHRPs, check with your COHRP contact(s).
Is this the end?
Yes! If you have ANY questions about ANY part of this process, please reach out to the USC IRB at hrpp@usc.edu for help!


