Post Approval Monitoring is designed to verify that research is being conducted as approved by the IRB, and help ensure compliance with federal, state, and institutional policies. IRB’s have oversight as long as the study is open. PAM serves to improve human research protections and the quality and integrity of research under the oversight of the USC IRB and has three goals:
- Enhance protection of research participants.
- Improve quality of human research data.
- Provide education to maintain compliance with HRPP regulations and policies.
The IRB may contact the Principal Investigator to assess the study using Post Approval Monitoring data collection via our REDCap form, consent document review, consent process review, or any other study related activity. The USC HRPP will be supporting the PI and study teams throughout the process. It is the goal of the HRPP for the PAM process to further the research mission of USC and provide education and knowledge.
For more information about the PAM, please see our Post Approval Monitoring policy, Chapter 17.3.
USC has multiple Post Approval Monitoring strategies:
- Self-assessment
- For Cause Audit
- Not for cause audit
- Progress Updates
The HRPP will notify the PI via email with a link to REDCap to address questions regarding Post Approval Monitoring. PI’s will have 30 business days to complete the questions within REDCap. If PI’s do not respond to the HRPP this will be considered noncompliance with our policy and may result in a hold on future submissions to the IRB, a full audit, or involvement of the Associate VP of Research Administration.
After receipt of the information requested via REDCap, the HRPP staff will review the information and will follow up with investigators within 15 business days with any additional requests, clarifications, interview requests, and possible site visit. Within 30 business days the HRPP will have a case report sent to the PI.
The questions that will be sent to investigators will address questions regarding the protocol, consent forms and supporting study documents. In preparation of addressing the questions it is important to carefully and objectively review your approved protocol and ensure that all staff are carefully following all activities as described and approved by the IRB. All IRB study procedures must be followed and if any changes are needed to the approved protocol, an amendment must be submitted and approved. The HRPP cannot stress the importance of following the approved protocol. Even small changes from the approved protocol may be of great concern to the IRB, federal regulatory agencies, and internal and external auditors.
The goal of PAM is to help support the research team, and facilitate research by ensuring that the approved IRB protocol and University Policy are being followed. The HRPP can also help researchers by identifying deficiencies so that the research team can put a plan in place for success and correct any deficiencies.
Upon completion of a PAM activity, the HRPP will prepare a case report that will be sent to the investigator and submitted both to iStar and RedCap. The case report will provide study teams with an overview of the findings and any potential follow-up actions that may be needed. Findings may include:
- No issues found and no action required.
- Recommendations or suggestions for consideration by the PI, such as sharing current best practices.
- Additional education for the principal investigator and/or study staff.
- Items requiring action – a request that an amendment or unanticipated problem be submitted.
- Forwarding to IRB for consideration of non-compliance findings.
If a concern or complaint about the conduct of a project is discovered or reported to the HRPP staff, any member of the IRB, or other administrative official, an audit for cause may be initiated. Audits for cause may occur at any time. The determination of the need for an audit for cause shall be made by the IRB Chair, HRPP QA/QI Team, Director, or HRPP Staff. An audit may be project-oriented (focused on a specific project) or research-oriented (focused on all the projects of a particular researcher). The PI of a project that has been selected for an audit for cause shall be notified at least one (1) working day in advance of the audit.
On site Assessment may be conducted by HRPP Staff. The role of the HRPP staff is to observe research activities, assist and educate the PI and research team by identifying deviations from the approved protocol. The HRPP staff will help identify who may be the best person to help with implementation of any required changes, and will be responsible for documenting the findings of the PAM assessment.
This form is for researchers to use to conduct self-assessments of their IRB approved projects to ensure they are being conducted in compliance with the approved procedures and IRB regulations and policies. The USC IRB requires researchers to conduct self-assessments at least annually for any project that lasts longer than one year, and may request self-assessments be reported to the USC IRB. Please keep copies of completed assessments with your IRB related records.
Some of the potential benefits that may be gained from a self-assessment include but are not limited to:
- Increased understanding of HRPP regulations and policies.
- Identifying areas of strength and/or need for improvement in research practices.
- Increased communication with IRB staff.
The IRB estimates that the self-assessment will take ~ 1 hour to complete or less.
- The IRB-approved version of the informed consent or assent document was not used.
- No informed consent obtained prior to study procedures.
- The informed consent document on file is not complete (i.e., only the page containing the signature is on file).
- Dates on informed consent documents for participant and researcher are not the same.
- CITI training for study personnel is expired or is not on file.
- Study personnel do not have a current conflict of interest disclosure on file with the COI office.
- Study documents not stored as indicated in the approved protocol OR stored with a linking list.
Progress updates will be used for all exempt and expedited studies that were not assigned a continuing review by the IRB. USC is committed to best practice. The Progress Update will provide the USC HRPP with information about study status. Progress Updates are not continuing reviews, but part of post approval monitoring. Investigators will receive auto acknowledgement regarding the Progress Update.
PAM FAQs
PAM Stands for Post Approval Monitoring. USC Human Research Protection Program (HRPP) is using RedCap to collect information about your study needed for PAM.
You will receive a request via iStar message. Please make sure when filling out the PAM tool that you are referencing the study where the request came from (this is important for investigators that have multiple studies open).
The PAM tool is in REDCap because it is a safe and secure way to manage sensitive data and allow you to save your work along the way.
There could be many reasons that you are being asked to fill this out. For example:
- Specific funding agencies (like the Department of Defense) may reach out the HRPP to audit studies.
- The IRB committee may request an audit of specific studies.
- Studies seen by the full committee may randomly be assigned to an audit so the HRPP can meet determine how to best support study teams by ensuring that activities are compliant.
This will depend on the organization of the study team. The HRPP staff are estimating between 30 minutes – 2 hours.
- Study Number as indicated by iStar (Please remember, if you have multiple studies, make sure that you are submitting the PAM tool for the studies requested by the HRPP via iStar).
- Study title.
- Name of Principal Investigator.
- If your study has informed consent forms (ICF) (expedited or full board studies) you will need to have the last 3 signed ICF’s to upload into the RedCap system.
No problem! You can return to the REDCap PAM tool as many times as you need to.
If your study was exempt we understand you do not have Informed Consent Form (ICF). There is no need to submit copies of your information sheets. Please fill out the PAM questions to the best of your ability.
Failure to complete PAM may result in being reported to Office of Culture, Ethics and Compliance (OCEC). If your study is federally funded OHRP/FDA may be notified for noncompliance.
Please reach out to the HRPP Quality Improvement Unit (QIU) at hrppqiu@usc.edu.
Progress Update FAQs
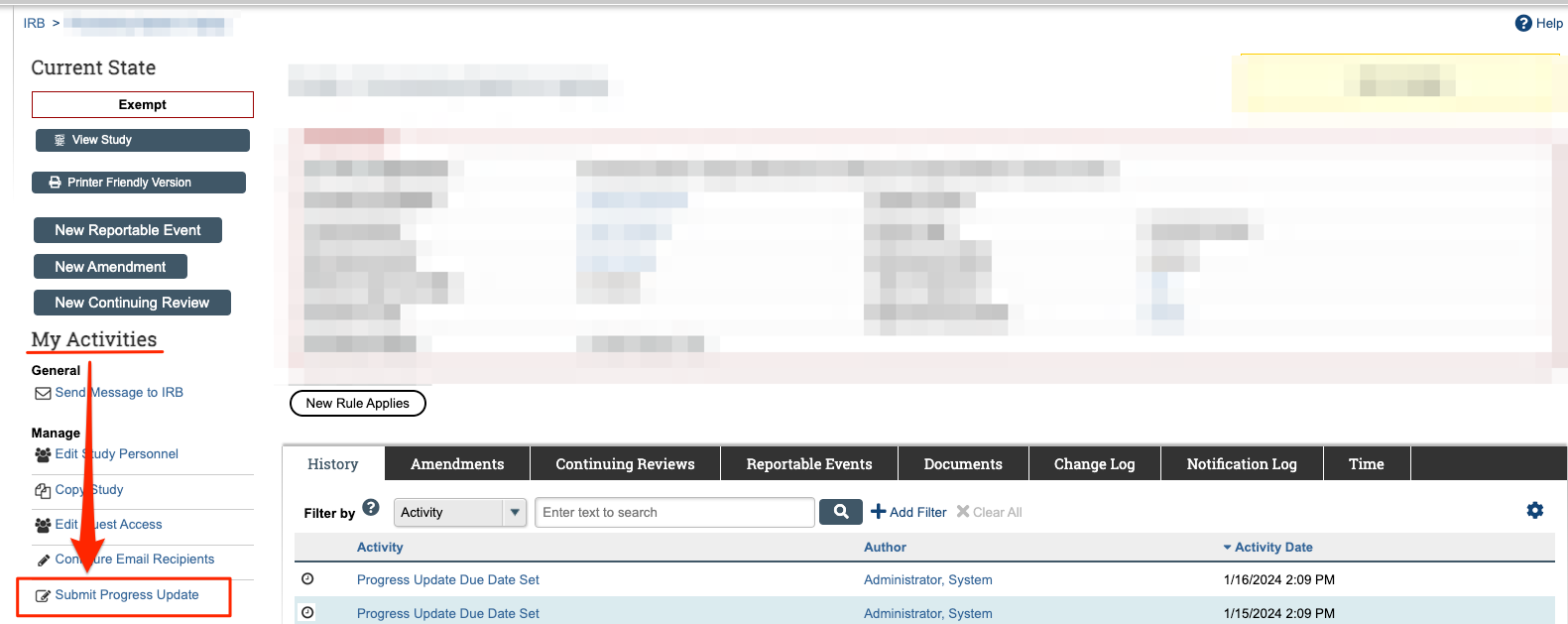
The Progress Update will be available as an activity you can execute iStar. You will find this on the left side of your screen. The activity will be available for execution under the “Manage” subheading under “My Activities” (on the left side) of the webpage as “Submit Progress Update.”
If you are not able to see the progress update activity, please reach out to the iStar Help Desk.
If all study activities have been completed and you would like to manually close out the study, please refer to this document for support on how to close an open study.
Anyone who received the notification is listed as personnel on the study and can close the study.
Please withdraw the amendment and then you will be able to close the study. You will find red button that says “Withdraw Amendment” on very left side of the screen when you scroll to the bottom.
All of the studies that require a progress update are available to view on your dashboard. Scroll down below “PI Staff Dashboard.” Next, please click the “Expiring/Expired” tab. You will find the “Expiring/Expired” tab to the right of your “My Inbox” in the middle of the page. This will list all the studies that need a progress update.
If you would like to keep the study open and active, please submit the progress update. You will find this on your iStar Dashboard for the specific study. The Progress Update will be available as an activity you can execute iStar. You will find this on the left side of your screen. The activity will be available for execution under the “Manage” subheading under “My Activities” (on the left side) of the webpage as “Submit Progress Update”. Please follow the link in the notification by clicking on the study ID to access iStar for this study and complete the Progress Update. You can also use the link in this notification to get directly to the study.
These were sent to you in error. We apologize and there is no action on your part.