sIRB Helpful Videos
The USC IRB will agree to serve as the sIRB for non-exempt multisite research and a maximum of 4 external US Relying Sites when:
- USC is the prime awardee of an NIH award and sIRB is required
- A federally funded multi-site research study requires sIRB per the funder (45 CFR 46.114)
- USC holds the IND on an investigator-initiated FDA regulated study on a case-by-case basis
NOTE: Single IRB does not apply to international sites. International sites should obtain their own IRB approval.
USC SINGLE IRB PROCESS
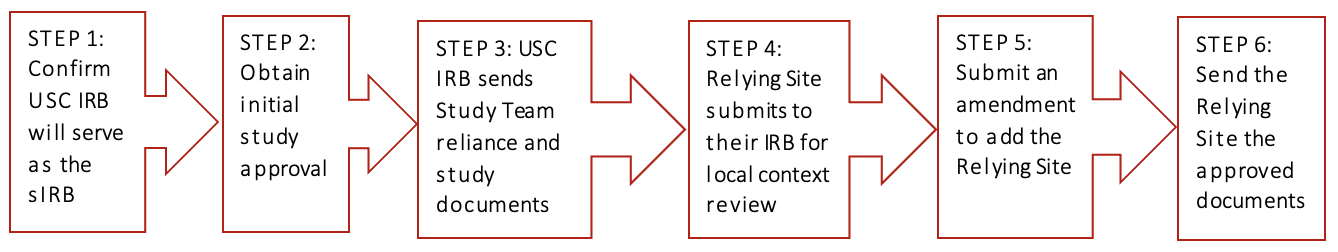
The USC Principal Investigator (PI) must contact the USC IRB Reliance Team at reliance@usc.edu and request confirmation that the USC IRB is able to serve as the single IRB of record. The USC PI should provide information about the funder, a copy of the protocol or grant submission, and the names of the participating sites.
A USC PI who agrees to become the Overall PI/Lead Study Team for multiple sites is responsible for the protection of human subjects for all Relying Sites in the study. Critical evaluation should take place to assure that the necessary resources and infrastructure are in place to support and lead a multi-site research project. Considerations include the management and collection of all regulatory information needed by funders/sponsors and/or regulatory bodies such as the FDA and a communication plan necessary to disseminate and receive all study-related matters between all sites.
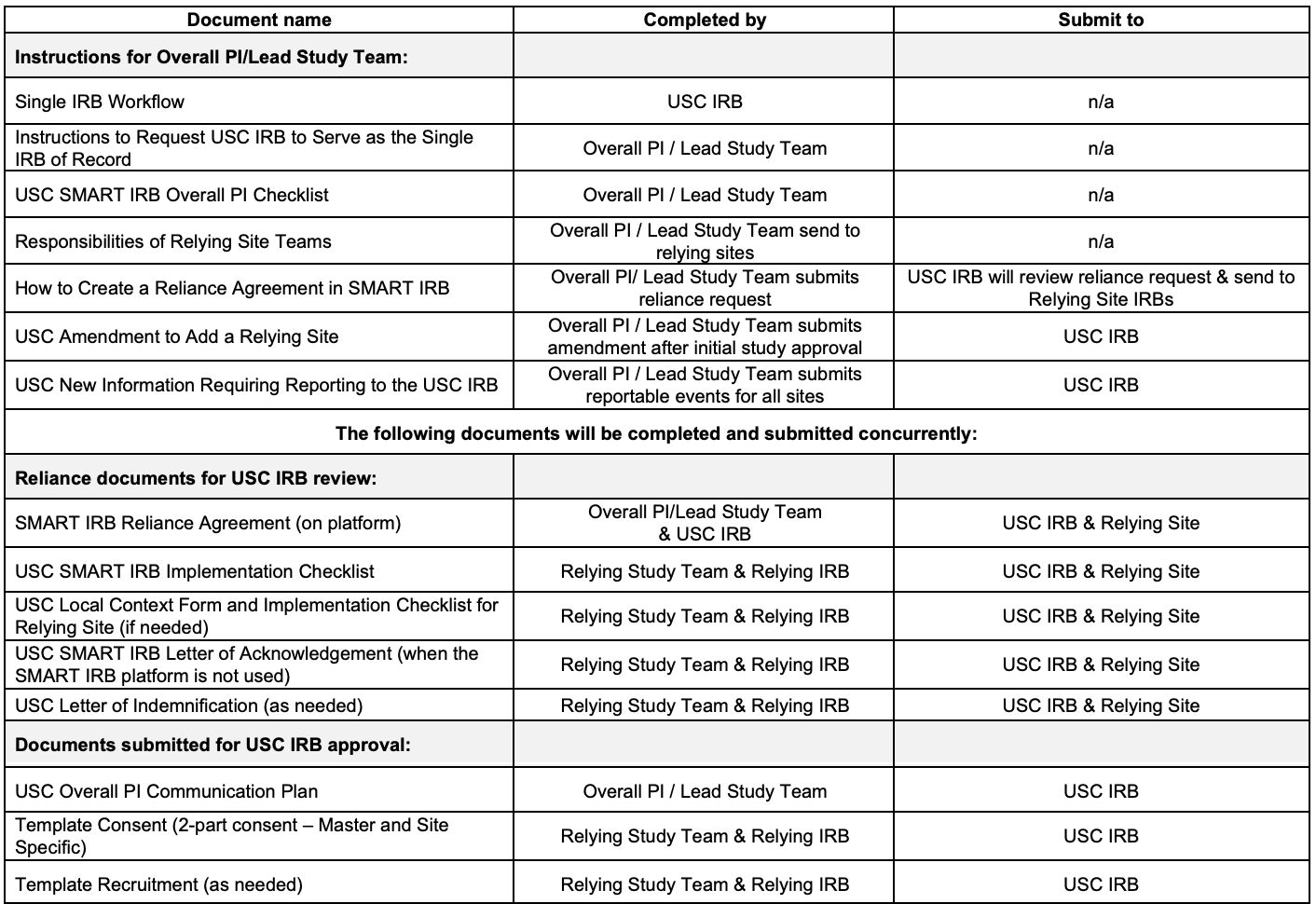
The USC Overall PI/Lead Study Team will need to review the following documents to understand the sIRB process and their responsibilities:
- Instructions to Request USC IRB to Serve as the Single IRB of Record
- USC Overall PI Communication Plan
- Single IRB Workflow
- How to Create a Reliance Agreement in SMART IRB
- SMART IRB Reliance Checklist
- USC SMART IRB Local Context Form and Implementation Checklist
- USC SMART IRB Overall PI Checklist
- USC Amendment to Add a Relying Site
- USC Reportable Events: New Information Requiring Reporting to the USC IRB
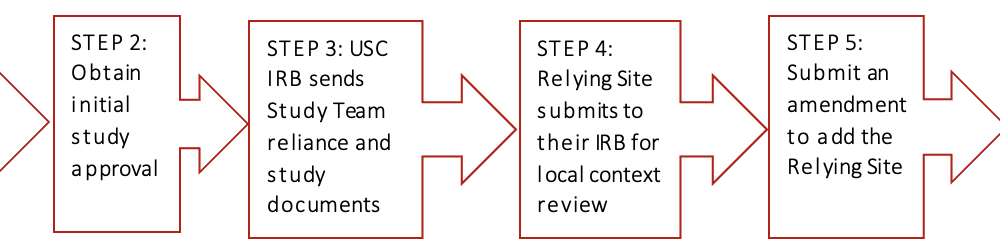
The study must be submitted to the USC IRB via iStar for initial review and approval. The submission should include the protocol, consent forms, recruitment documents, USC Overall Principal Investigator’s Communication Plan, and any other study materials for USC IRB review (i.e., data collection sheets, questionnaires, drug package inserts/investigator’s brochure, device information, etc.). NOTE: Participating sites should NOT be added to the initial study application. Once the study is approved, each participating site should be added via individual amendment.
The USC IRB uses the SMART IRB platform to establish the reliance agreement with the Relying Site. Any exceptions to using SMART IRB must be discussed with the USC IRB reliance team. The reliance request should be submitted on the SMART IRB platform for the entire study. All the participating sites should be listed on the reliance request if there is more than one Relying Site. Additional Relying Sites may be added after the original reliance agreement is completed by submitting an amendment to the agreement on the SMART IRB platform. The USC IRB approved protocol and consent should be uploaded to the SMART IRB reliance request. The USC IRB will send the Overall PI/Lead Study Team the reliance documents and consent form templates to be completed by the Relying Site and their IRB.
The USC Overall PI/ Lead Study Team must provide the Relying Site with the following documents:
- The USC IRB approval letter
- The USC IRB approved protocol
- The USC IRB approved consent template form (if applicable)
- The USC IRB reliance documents for the relying site to complete and sign (provided by USC IRB Reliance Team)
- Any other reliance documents required by the USC IRB
The Relying Site should follow their institution’s procedures for relying on another IRB. Their IRB will need to conduct a review to ensure that all local requirements have been met. This is called a local context review. Further information should be obtained from the Relying Site’s IRB office.
The Overall PI/Lead Study Team will submit an amendment via iStar to add a Relying Site. Only one site should be added per amendment. The amendment should include the reliance documents completed by the Relying Site and their IRB, the consent documents, recruitment documents, and any other documents for USC IRB review.
The investigators at the relying site should provide the USC Lead PI/Lead Study Team with the following documents to submit to the USC IRB:
- The Relying Sites IRB communication that the local context review and all ancillary reviews are completed
- The Relying Site reliance forms, consent forms, and recruitment documents
The USC Overall PI/Lead Study Team should provide the Relying Site with the following documents:
- The USC IRB approval letter of the Relying Site
- The USC IRB approved Relying Site consent forms (if applicable)
- The USC IRB approved reliance documents (provided by USC IRB Reliance Team)
- Any other documents approved by the USC IRB
Links to sIRB documents:
Instructions to Request USC IRB to Serve as the Single IRB of Record 8.26.2022
- Appendix 1 Single IRB Workflow
- Appendix 2 How to Create a Reliance Agreement in SMART IRB
- Appendix 6 USC SMART IRB Overall PI Checklist
- Appendix 7 USC Amendment to Add a Relying Site v06-09-2022
- Appendix 11 USC Overall PI Communication Plan 06-06-2022
- Appendix 13 Responsibilities of Relying Site Teams