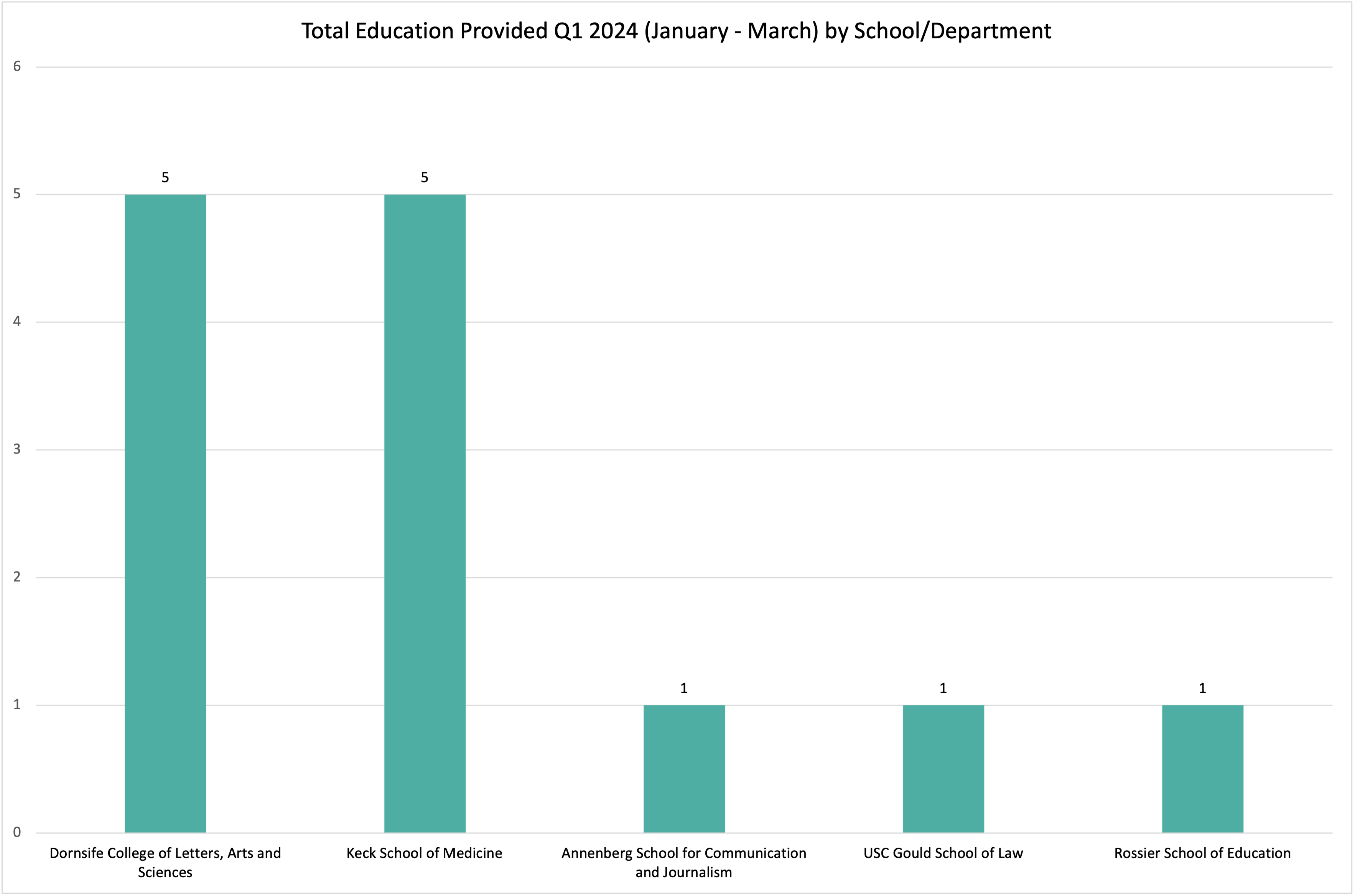
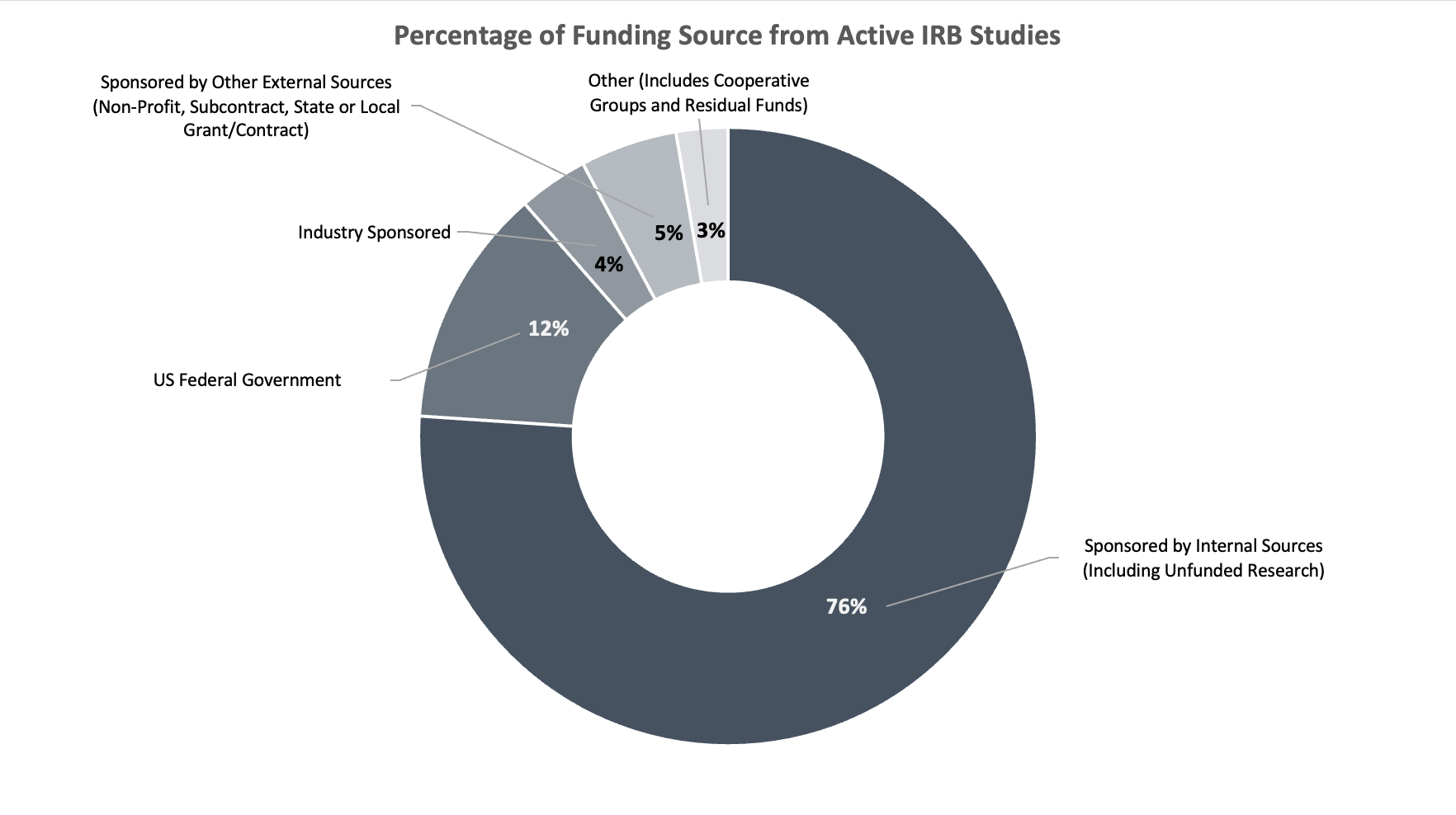
The HRPP is committed to promoting the adherence to and enactment of the Belmont Report principles of Respect for Person, Beneficence, and Justice. The Quality Improvement Unit (QIU) of the HRPP supports continuous quality improvement activities to foster the ethical treatment and protection of human participants in research. Quality improvement activities continue our commitment of transparency with the USC community and are associated with the Association for the Accreditation of Human Research Protection Programs (AAHRPP) standard I-5.
STANDARD I-5: The organization measures and improves, when necessary, compliance with organizational policies and procedures and applicable laws, regulations, codes, and guidance. The organization also measures and improves, when necessary, the quality, effectiveness, and efficiency of the Human Research Protection Program.
IRB Wait Time
Our homepage depicts the approximate time it takes for an IRB Analyst to be assigned to review your new exempt or expedited protocol submission. Data is refreshed on Monday of every week. Please refer to our Getting Started page and IRB Submission Guidelines to ensure that studies submitted are ready for review and positioned for appropriate action.
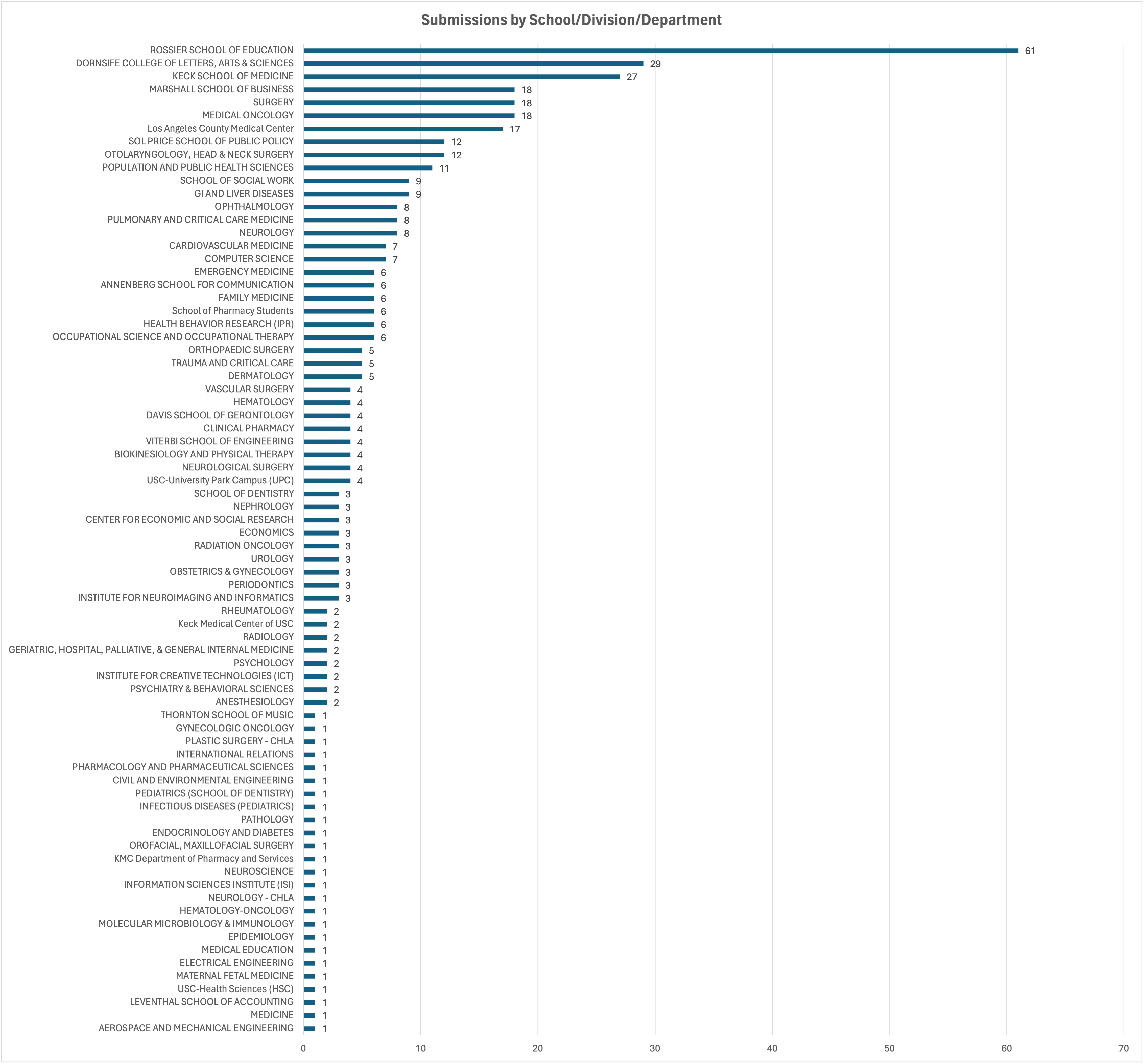
The Quality Improvement Unit (QIU) of the Human Research Protection Program (HRPP) conducted a five-year analysis of total IRB submissions.
The HRPP publishes a dashboard every fiscal year (July 1–June 30) on IRB performance and metrics.
- Fiscal Year 2021–2022
- Fiscal Year 2022–2023
- Fiscal Year 2023-2024 (Released in July 2024)
Data will be provided at the end of Q2 (June), after the completion of progress updates.