Welcome to the USC IRB! Below is a list of resources to help you get started for IRB review. Click on the common questions and topics below to learn more about the process.
An activity requires IRB review if it fits the federal definition below for research and human subjects or the FDA definitions of clinical investigation and human subjects.
- Research means a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.
And
- Human subject means a living individual about whom an investigator (whether professional or student) conducting research:
- Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or
- Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.
Or
- Meets the FDA definition of clinical investigation and human subject.
Clinical investigation means any experiment that involves a test article and one or more human subjects, and that either: a) must meet the requirements for prior submission to the Food and Drug Administration; or b) the results of which are intended to be later submitted to, or held for inspection by, the FDA as part of an application for a research or marketing permit. The terms research, clinical research, clinical study, study, and clinical investigation are deemed to be synonymous. Note: The DHHS regulatory definition of research is different.
Human subject, under FDA regulations, means an individual who is or becomes a participant in research, either as a recipient of the test article or as a control. A subject may be either a healthy individual or a patient. For device studies, this includes an individual on whom or on whose specimen an investigational device is used. Note: The DHHS regulatory definition of a human subject is different.
Not Human Subjects Research (NHSR)
USC policy allows researchers to make a NHSR determination themselves if a project does not meet the regulatory definition of human subjects and/or research. Please view the Not Human Subjects Research (NHSR) page for guidance.
Who may serve as a Principal Investigator
Human research studies require an appropriately qualified individual to serve as the Principal Investigator (PI). A PI must be identified before the IRB will review an application. More information can be found in our policy.
iStar is the USC system for review of human participant research, animal research, radiation safety and biosafety. The USC Institutional Review Boards use iStar to review studies that require IRB review and to certify studies that do not require IRB review.
Go to iStar | istar.usc.edu
Please login to iStar with your USC NetID. If you do not currently have an account, one can be automatically created upon login.
Please contact the iStar Help Desk at iStar@usc.edu for account access inquiries.
For an application to be approved by the USC IRB, all study personnel must complete Human Subjects (HS) research training. Additional training may be required based on study specific factors. The online course used (unless otherwise stated) is provided by the Collaborative Institutional Training Initiative (CITI) at CITI Program. Please refer to the CITI FAQs for further guidance on obtaining an account and adding a course.
Please refer to the Education & Certification page for a list of required courses.
Trojan Learn Curriculum
USC researchers and IRB members share responsibility for ensuring that human research conducted under USC’s jurisdiction meets the ethical principles of the Belmont Report and is compliant with federal regulations and university policy.
Trojan Learn offers education and training for USC faculty and staff on a number of topics related to human research and the IRB submission process. To access these trainings, visit Trojan Learn, log in with USC credentials, and paste the following course titles into the search bar. Below is a list of available courses, organized by the offices that developed each course.
USC Office of Research and Innovation
- IRB Protocol Template: Best Practices for a Successful Submission (45 minutes)
- This course is intended for anyone who may be submitting to the Internal Review Board (IRB), including faculty, staff, and students. The required IRB Protocol Template is a detailed outline of the proposed research study. This training will present the general components of an IRB Protocol Template and what information is expected for each of these components.
USC Contracts and Grants
- Grants Management Training for Staff (2 hours, 45 minutes)
- The responsibilities of investigators and research administrators are rapidly evolving and maintaining up to date knowledge in the relevant critical area is often difficult. In recognition of these obligations and as part of USC’s continuing desire to remain consistent with our peers in the institutional research community, the University has developed this Grants Management Training Program for Staff. This two-course program is mandatory for all research administrators and other support staff who are seeking expenditure authority on accounts. The courses in this training program are cross-listed with the Cardinal & Gold Curriculum. Progress and completions count for both programs.
- Grants Management Training for Faculty Program (3 hours)
- The responsibilities of investigators and research administrators are rapidly evolving and maintaining up to date knowledge in the relevant critical area is often difficult. In recognition of these obligations and as part of USC’s continuing desire to remain consistent with our peers in the institutional research community, the University has developed this Grants Management Training Program for Faculty.
- The Cardinal & Gold Curriculum (11 hours, 51 minutes)
- The C&G (Cardinal and Gold) Curriculum is a comprehensive research administration training program developed to meet the needs of today’s evolving research environment. Our training program allows participants to: a.) develop higher competency, b.) increase efficiency, and c.) improve compliance according to USC and sponsor requirements. Instructional courses have been designed to foster an interactive learning environment while embracing new technologies and processes. Moreover, the certification program ensures a standard level of expertise among those who support the research endeavor at USC.
- Preparing Proposals in Cayuse Sponsored Projects (1 hour)
- This course will guide proposal preparers, approvers, and PI’s through the Cayuse SP interface. The program aims to assist researchers and research administrators in creating, routing, and approving proposal records through the Cayuse SP system.
- The Basics of Bayh-Dole (20 minutes)
- This course will cover the basics of the Bayh-Dole Act as well as the obligations of USC researchers under the Bayh-Dole Act.
- NCURA Life Cycle of the Award Series (22 hours, 1 minute)
- Research Administration in its many varied facets continuously evolves, and most universities and colleges are challenged to keep up with the increasingly complex, ever-changing environment. The National Council of University Research Administrators (NCURA) has developed a unique approach to meeting this challenge by providing this video webinar series. These webinars are designed to offer professional growth for grant managers and research administration personnel and span a wide range of topics including proposal development and budgeting, award negotiation and acceptance, award management and compliance.
Keck Medical Center of USC
- Introduction to KECKCap (10 minutes)
- The Clinical Data Pull (CDP) feature allows investigators to automatically extract real-time patient medical record data into KECKCap, which is USC Keck’s clinical instance of REDCap. CDP can be used in research studies using patient medical record data or for projects that support clinical care. This course will provide an overview of CDP, teach users how to use CDP, and explain users’ responsibilities when using CDP. Users are required to complete this course before requesting access to the CDP feature.
- Using KECKCap (20 minutes)
- The Clinical Data Pull (CDP) feature allows investigators to automatically extract real-time patient medical record data into KECKCap, which is USC Keck’s clinical instance of REDCap. CDP can be used in research studies using patient medical record data or for projects that support clinical care. This course will provide an overview of CDP, teach users how to use CDP, and explain users’ responsibilities when using CDP. Users are required to complete this course before requesting access to the CDP feature.
- Compliance (5 minutes)
- The Clinical Data Pull (CDP) feature allows investigators to automatically extract real-time patient medical record data into KECKCap, which is USC Keck’s clinical instance of REDCap. CDP can be used in research studies using patient medical record data or for projects that support clinical care. This course will provide an overview of CDP, teach users how to use CDP, and explain users’ responsibilities when using CDP. Users are required to complete this course before requesting access to the CDP feature.
- Extracting Medical Record Data Through Clinical Data Pull (CDP) in KECKCap (35 minutes)
- The Clinical Data Pull (CDP) feature allows investigators to automatically extract real-time patient medical record data into KECKCap, which is USC Keck’s clinical instance of REDCap. CDP can be used in research studies using patient medical record data or for projects that support clinical care. This course will provide an overview of CDP, teach users how to use CDP, and explain users’ responsibilities when using CDP. Users are required to complete this course before requesting access to the CDP feature.
USC Office of Culture Ethics and Compliance
- CMMC Security Requirements for DoD Funded Projects (recording) (55 minutes)
- Presentation by Daniel Shapiro, Associate Vice President for Research Compliance in the Office of Culture, Ethics, and Compliance. Overview of the Cybersecurity Maturity Model Certification (CMMC) and the associated restrictions found with these projects.
- NIH Data Management and Sharing Requirements (recording) (1 hour)
- Effective January 25, 2023, the NIH expects that investigators and institutions comply with the Data Management and Sharing (DMS) policy. Under the DMS policy, investigators and institutions must: plan and budget for the managing and sharing of data; submit a DMS plan for review when applying for funding, and comply with the approved DMS plan. The research covered by the 2023 Data Management & Sharing Policy includes: Competing grant applications that are submitted to NIH for January 25, 2023, and subsequent receipt dates; Proposals for contracts that are submitted to NIH on or after January 25, 2023; NIH Intramural Research Projects conducted on or after January 25, 2023; and other funding agreements (e.g., Other Transactions) that are executed on or after January 25, 2023, unless otherwise stipulated by NIH. For additional resources, please visit the Department of Contracts and Grants website https://dcg.usc.edu/nih-data-management/
- USC HIPAA Compliance and You (45 minutes)
- In this course, you’ll learn about the Health Insurance Portability and Accountability Act (HIPAA) by reviewing the basics of the Privacy, Security, and Breach Notification Rules. Throughout, you’ll be presented with real-life examples to guide you through the dos and don’ts so you can confidently protect patients’ personal health information in your role at USC. You’ll also learn to recognize violations and report breaches.
- USC FERPA Awareness (30 minutes)
- The Family Educational Rights and Privacy Act (FERPA) of 1974 is a federal law regarding the privacy of student education records and establishes requirements related to the release of the records and the access provided to the records. As a leading educational institution, it is important that the Trojan community is aware of the USC FERPA policy and practices related to student education records. By taking this course, you will gain information on how to respond to student privacy issues and what you can do to protect the privacy of student information.
- Safeguarding Confidential Data (15 minutes)
- This course is required for individuals at USC with heightened access to confidential data, such as student, employee, health, and financial data. It covers data protection laws, USC policies, and best practices for handling sensitive data.
- HHS Conflicts of Interest in Research
- In 2012, the Department of Health and Human Services (HHS) issued revised conflict of interest regulations. Effective August 24, 2012, researchers who seek or have obtained HHS support (including NIH, CDC, HRSA, AHRQ and other HHS agencies) must disclose all financial interests related to their institutional responsibilities to USC on an annual basis. HHS also requires training to be taken at least every four years. This training satisfies that requirement.
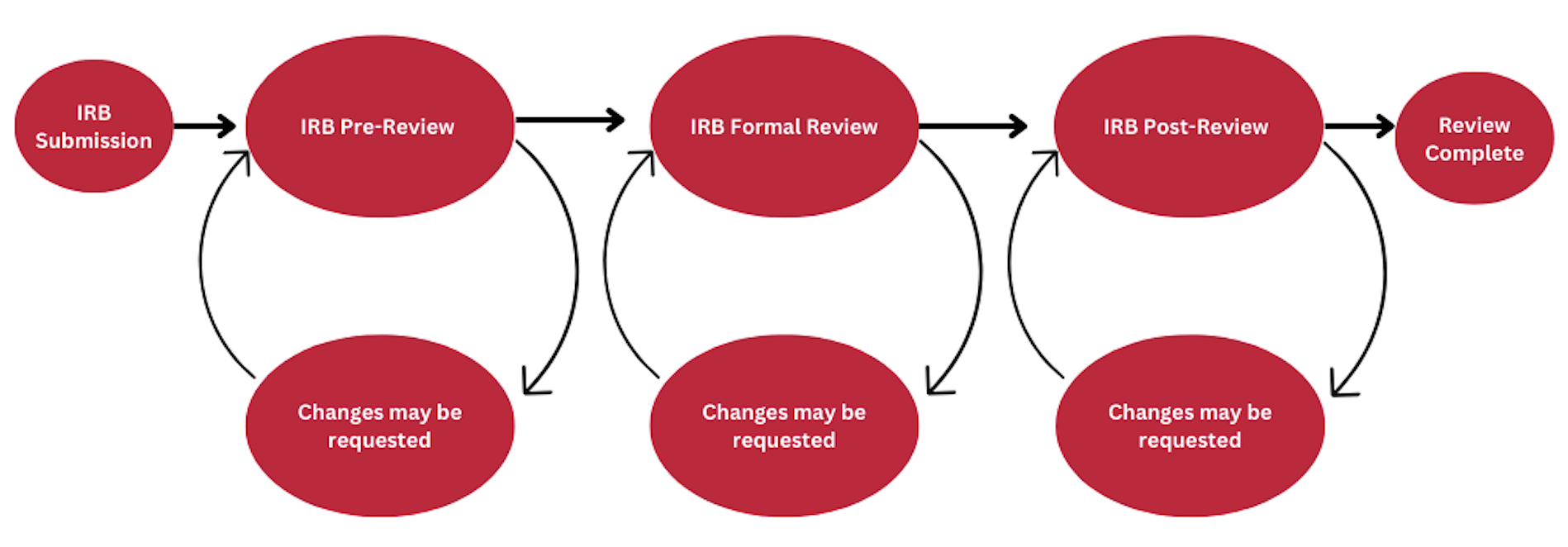
To submit an application to the IRB, an appropriate level of review must be requested. The IRB will verify or correct the level of review requested for an IRB submission.
Drop into Ask an Analyst for additional support.
Login to your iStar account and click on “Create a New IRB Study.” The iStar system uses a smart form, which will populate questions based on the responses. iStar questions that are marked with a red asterisk (*) require a response from you.
To ensure all submissions to the USC IRBs are reviewed efficiently and thoroughly, review IRB Submission Guidelines prior to submission.
- Stacking documents is necessary to properly keep track of documents in iStar. Please view our guidance on how to stack documents.
- Learn about the circumstances that may meet the requirements for urgent review.
- Guidance on starting a research trial for both Biomedical and Social Behavioral research at USC.
- Drop into Ask an Analyst for additional support.
- Guidance for student researchers and faculty advisors.
Investigators who want to serve as the Lead PI for a multi-site study should review the Requesting USC IRB to Act as sIRB
Investigators who want to participate in a multi-side study and cede IRB review to an external Central/Single IRB should review the Requesting USC to Rely on an External IRB page.
Please review the multi-site IRB FAQs.
If your study is funded by the DoD, please visit our Department of Defense guidance page.
The USC IRB provides protocol, consent, and authorization forms and templates along with other helpful documents on the Forms and Templates page.
Please note that the use of an incorrect protocol will result in the application being returned without review.
IRB Review Process
IRB Review: How to