- NOTE: If the sIRB has determined the study to be “Exempt,” you should submit an Exempt Review application to the USC IRB and not a Ceded Review application.
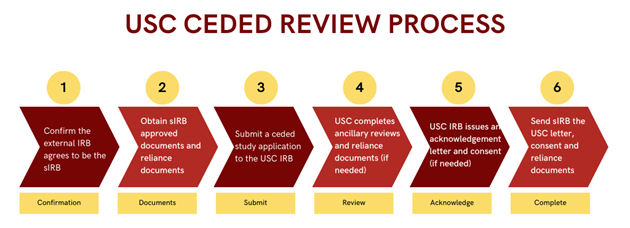
The sIRB should confirm in writing that they are willing to serve as the sIRB. This may be done by email, communication from a sponsor/funder, or via the reliance request on the SMART IRB platform.
The sIRB, Overall PI/Lead Study Team, Sponsor or Clinical Research Organization (CRO) should provide the USC PI/Study Team with the following sIRB documents:
- The sIRB study approval letter listing the determinations made for the study and the submitted study documents
- The sIRB approved protocol
- The sIRB approved consent template as a Word document (if applicable)
- Any reliance documents required by the sIRB
You must submit a ceded study application via iStar. The submission of the ceded study application will allow the USC IRB to conduct a local context review to confirm that the researchers are qualified to conduct the study at USC and that the study meets all institutional, local, and state requirements. The USC IRB will also determine whether other ancillary reviews are required at USC before the study is conducted (i.e., Clinical Trials Office, Department of Contracts & Grants, Biosafety Committee, Radiation Safety Committee, Conflict of Interest Review Committee, etc.). The USC IRB will review the ceded study application to ensure that all local requirements have been met. This is called a local context review.
- In section 1.1, select “Rely on another IRB (Ceded).” You should select the appropriate USC IRB in the application based on the type of research being conducted, biomedical or social behavioral. It is important to select the correct USC IRB since it may be noted in the reliance agreement and the appropriate IRB Chair may need to review any reportable events required to be submitted to USC. See the HRPP website for more information on the types of research https://hrpp.usc.edu/research/.
- In section 2.1, list all site personnel who will be involved in conducting the research and their roles in the study.
- In section 4, indicate the type of funding and respond to all questions. The details of the funding should be provided in section 4.4 and the funding should be linked to Cayuse in section 4.5.
- In section D1.1, indicate the name of the reviewing IRB. The name should match what is listed in the IRB approval letter.
- In section D1.2, upload the sIRB approval letter, approved study protocol, approved consent template (if applicable), and any reliance documents (such as local context document and forms that require additional signatures) to the ceded study application. You should also submit the initial IRB approval if the version of the protocol you are submitting is not the initial version so the IRB can review any determinations made by the reviewing IRB.
NOTES: If Advarra or WCG IRB is the external IRB, you do not need to upload the master reliance agreement. If the SMART IRB platform is used for the reliance agreement, a determination letter will be issued when the reliance agreement is executed. You will need to upload the determination letter for the reliance agreement, in section D1.2.
If LA General Medical Center is a participating site and a letter of indemnification is requested from the reviewing IRB, they should remove the indemnification provision since the Los Angeles County Department of Health Services will not sign the letter.
- In section D1.3, state which study activities will be done by the study site personnel. You should also note any differences in the study activities or study populations at your study site.
- In section D1.5, the selections made in this section should be consistent with the activities listed for the study personnel in section 2.1.
- In section D2.1, note any medical procedures that require ancillary review, such as IBC or RSC. This will populate a section to link to those applications. Ancillary reviews may be submitted concurrently with the ceded study application. All ancillary reviews must be completed before the USC IRB completes the local context review.
- In section 24.7, upload the USC consent. See below for further instructions on how to create the USC consent.
- In section 25, the cost, payment, and injury statements in this section should be listed in the consent. For industry funded studies, the USC Clinical Trials Office (CTO) will review the statements in section 25 and provide a consistency review. The language in the consent should match the language CTO provided in section 25. If the study has federal, foundation, or grant funding, this section may require review by the USC Department of Contracts and Grants (DCG) and they will also provide a consistency review. The language in the consent should match what is provided by DCG. For all other studies, the language in this section is the responsibility of the Principal Investigator and should match the language in the informed consent.
- In section 35.1, respond “Yes” if medical records will be used in the study and complete all of the sections that appear in the application.
- In section 36, in most cases the USC IRB will be the privacy board and make HIPAA determinations and grant any HIPAA waivers for the study. You should request and justify any HIPAA waivers in this section.
- In section 39.1, any conflicts listed in this section require review by the Conflict of Interest Review Committee (CIRC). See the website for more information about conflict of interest in research https://policy.usc.edu/conflict-of-interest-in-research/.
- In section 45, you should select all of the USC and LA General Medical Center locations where the research will be conducted.
If a USC consent is needed, you should create the USC consent by revising the sIRB consent template using tracked changes to add required USC language. Do not create the USC consent using the USC IRB consent template.
The USC required language is listed in the Informed Consent Form Instructions for Industry Sponsored, Cooperative Group, or External IRB Studies located on the HRPP/IRB website Forms and Templates page.
The following applies to funded interventional studies:
Participants may not be enrolled into the study until the USC Clinical Trials Office (CTO) or Department of Contracts & Grants (DCG) has provided final cost, payment, and/or injury language for the USC consent form. The USC consent form must be revised to be consistent with the final language, if needed, and reviewed by the USC IRB. The revised USC consent form must then be submitted to the sIRB of record for approval.
The sIRB may want more information about state and local laws, institutional policies, participating sites, participating study teams (e.g., training and conflicts of interests) and variations in study conduct (e.g., ancillary reviews required; HIPAA requirements; subject population). They may collect this information using a local context form. You should complete as much information as possible on the local context form. The USC IRB will complete any sections of the form that specifies that it should be completed by the IRB. The USC IRB will also review the completed form to ensure that the information is accurate and is consistent with the USC study activities listed in the protocol and the iStar application.
Below are the USC institutional profiles for Health Science Campus (HSC), University Park Campus (UPC), and LA General Medical Center (LAGMC) that includes information that may be used to complete the local context form.
IREx_ Institutional Profile – HSC updated 08-13-2024
The USC IRB will issue an acknowledgement letter for the ceded study application when all USC requirements are met, and all required ancillary reviews are completed.
You are required to submit the acknowledgement letter to Advarra and WCG IRBs when submitting the study for their review. They will not review the study if you do not provide the acknowledgement letter. When submitting to WCG IRB, you should add the USC IRB number to the “Institution Tracking No.” to easily identify the USC study.
You may submit the acknowledgement letter to other external IRBs if required by the reviewing IRB to confirm that all local requirements have been met.
NOTE: The investigator cannot conduct study activities until the reliance agreement is fully executed, USC IRB has issued an acknowledgement letter for the ceded study application, and if applicable, the contract is fully executed.
After the USC IRB issues the acknowledgement letter, you should send the sIRB/Overall PI/Lead Study Team the USC acknowledgement letter, USC consent (if applicable), and any reliance documents required by the sIRB.
The USC PI/Study Team is responsible for ensuring that:
- all USC requirements are met
- all required ancillary committee reviews are completed before and while conducting the study at USC
- communicating to the USC IRB any changes that require USC IRB review or review by USC ancillary committees
The USC PI/Study Team is also responsible for reporting the following in accordance with the Reviewing IRB’s policies and procedures for timing and content of such submissions:
- communicating with the Overall PI/Lead Study Team
- reporting any local changes to the study team or funding
- providing information for continuing review
- reporting reportable events that occur at USC such as adverse events, participant complaints, unanticipated problems, noncompliance, suspension, and termination
- providing access to study records for audit by the USC IRB, sIRB, or other regulatory or monitoring entities
You are required to submit an amendment to the USC IRB BEFORE submitting to the sIRB for the following reasons only. All other changes should only be submitted to the sIRB for review and approval.
- Addition or removal of investigators
- New conflict of interest for investigators
- Addition of special populations (adults who are not competent to consent or minors)
- Addition of Los Angeles Medical Center as a study location
- New consents
- Changes in HIPAA authorization forms or waivers
- Changes that require review by other USC committees (i.e., Clinical Trials Office, Department of Contracts & Grants, Biosafety Committee, Radiation Safety Committee, etc.) such as changes in funding or sponsor, cost/compensation/injury to participants, or addition of research procedures
A continuing review does not need to be submitted to the USC IRB. The continuing review will be submitted to the sIRB. You are responsible for providing all necessary information to the sIRB/Overall PI/Lead Study Team for the sIRB continuing review.
No expiration date will be set in iStar for the ceded study application since USC’s reliance on the sIRB will only expire when the study is closed.
NOTE: If the study is closed by the sIRB, the study should be closed in iStar and the sIRB study closure notice uploaded to the closure application.
You must report all reportable events, such as unanticipated problems, protocol deviations, participant complaints, and other reportable events to the Overall PI/Lead Study Team or sIRB according to the sIRB Standard Operating Procedure for reporting.
If the sIRB has made any of the following determinations for any reportable events, a reportable event must be submitted at USC: Noncompliance, Serious Noncompliance, Continuing Noncompliance, Unanticipated Problem (internal and external), Suspension, and Termination.
USC IRB also requires that all participant complaints be reported to USC IRB.
Submit all reportable events in iStar as soon as possible, but no later than 10 working days after you become aware of the event.
NOTE: Failure to report an event may result in the suspension of the study, and reporting to the required agencies, including funding agency, regulatory agencies (i.e., FDA, OHRP), etc.
For additional information please contact the USC IRB at reliance@usc.edu.

