Expanded Access and Right to Try Criteria
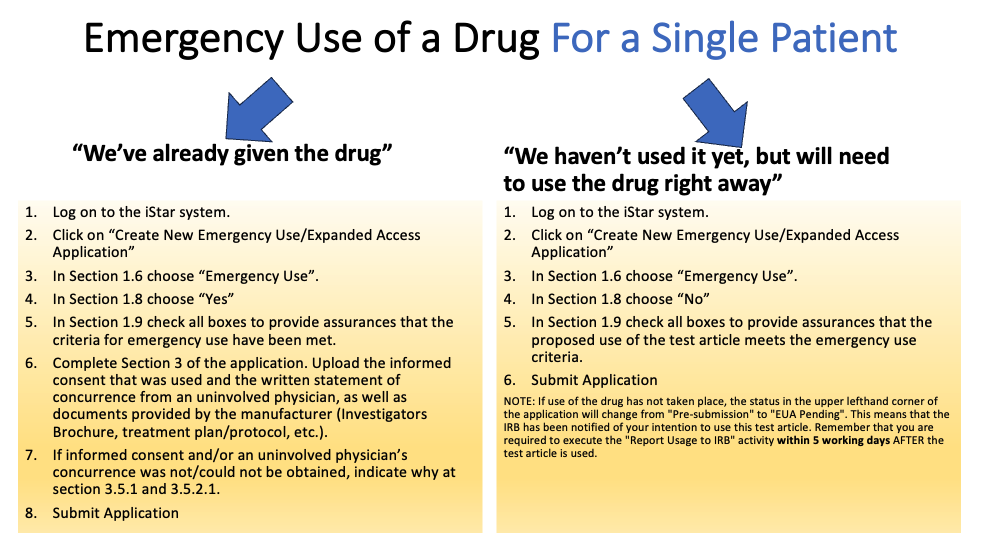
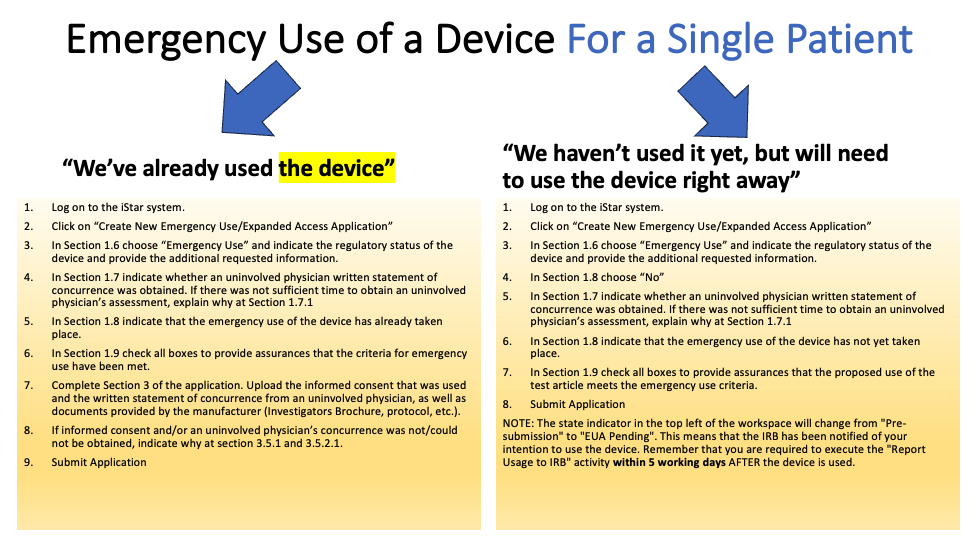
Emergency Use (the patient is in an immediately life-threatening situation)
- Must meet the FDA definition of life-threatening.
- Allows for the one-time use of an investigational drug or device.
- No time to get standard USC IRB approval before use of an investigational drug or device.
- Physician must submit an Emergency Use Application in iStar before the drug or device is used.
- FDA authorization is required before an investigational drug is used.
- For a device, there is no time to obtain FDA authorization or IRB approval due to the emergent nature of the patient’s condition
- Informed consent must be sought from patient or legally authorized representative.
- Independent physician must certify the use of the drug or device
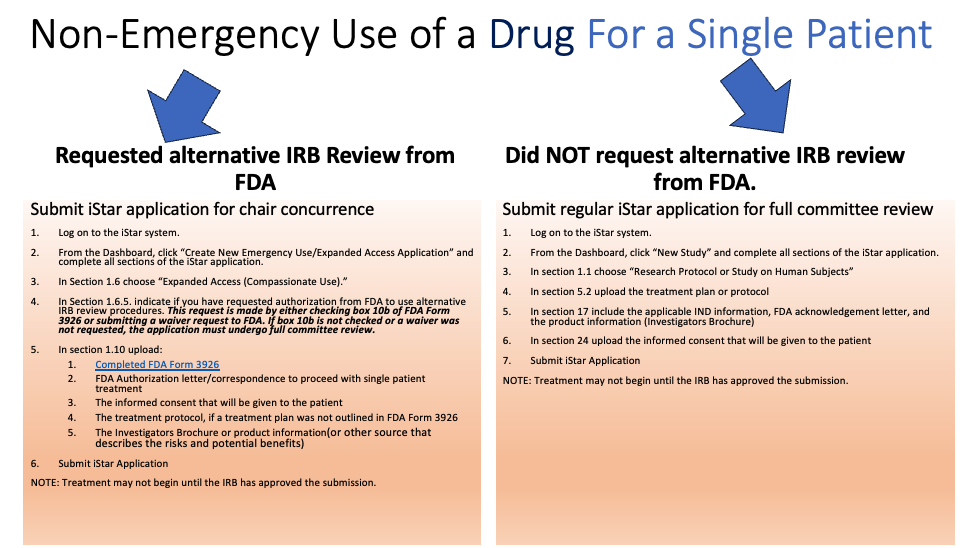
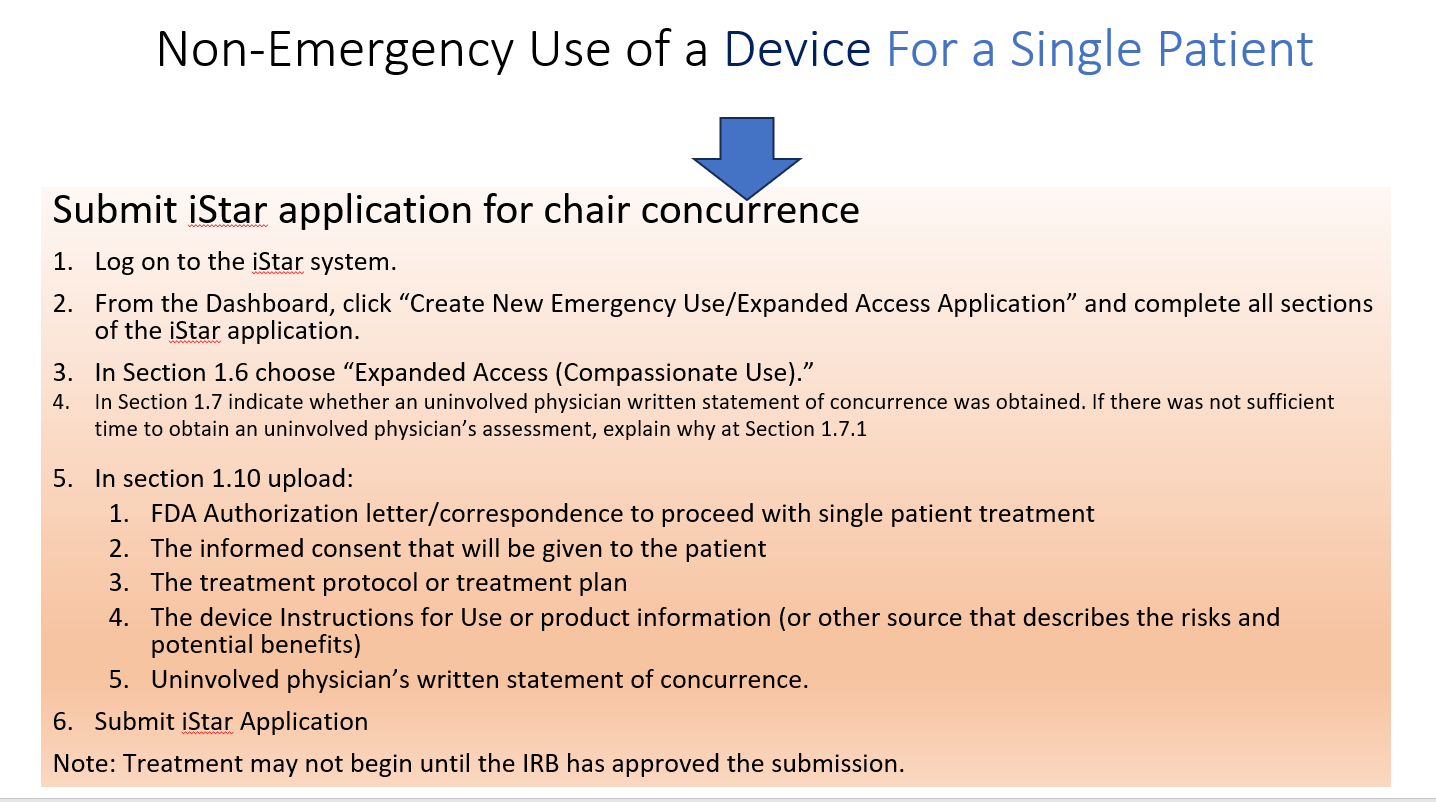
Non-Emergency Use (the patient has a serious condition and is not in an immediately life-threatening situation)
- IRB review and approval is required before the use of the investigational drug or device.
- Prior FDA approval is needed before non-emergency use of a device or drug occurs.
- Informed consent must be obtained
- Patient must have a life-threatening illness, no standard treatments available, not eligible for a clinical trial of the drug
- Allows use of an experimental drug outside of a clinical trial
- Unlike expanded access, does not require FDA approval
- IRB review and approval is required before the use of the investigational drug
- Only covers drugs; devices do not fall under Right To Try
- IRB review and approval is required
- Submit a Right To Try application using the regular iStar study application